Peng Wu Laboratory: Chemical Immunology
The research in the Wu laboratory integrates synthetic chemistry with glycobiology to explore the cellular and molecular mechanisms that control immune responses toward cancer and human pathogens.
The early work of our group was focused on the development of chemical tools to explore the relevance of protein glycosylation to human disease.
The glycome, defined as the full complement of glycans that a cell produces, is involved in a myriad of physiological processes, including angiogenesis, fertilization, stem cell development, and neuronal development. Changes in the glycome have also been shown to mark the onset of cancer and inflammation. Produced by the secondary metabolism rather than encoded in the genome, glycans are assembled in a stepwise fashion by multiple enzymes and thus by multiple genes. Consequently, genetic and biochemical tools alone cannot be used to define all aspects of the glycome. Therefore, my lab chooses to develop complementary chemical tools that can be applied in parallel to assemble a picture of the glycome both from the “bottom up” and from the “top down”.
Chemical tools developed by my lab include:
- The most efficient catalysts for Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC), the prototypical example of click chemistry, for labeling glycans and other biomolecules in vivo and for proteomic analysis, which have been used by more than 250 labs worldwide.
- Chemoenzymatic methods for the detection and modification of cell-surface glycans
Major discoveries enabled by these tools include:
- The discovery of fucosylated glycans as receptors for influenza virus infection besides sialylated glycans, the only previously known host receptors.
- Increasing N-glycan a1,3-fucosylation elevates the Wnt co-receptor Lrp6 endocytosis, leading to the suppression of Wnt-β-catenin signaling.
- Mutation of cloche, a gene responsible for the blood circling system formation, leads to the down‐regulation of fucosylation in zebrafish embryogenesis. Exogenously introduced fucose or GDP‐fucose partially rescues this phenotype and significantly increases the life spans of cloche mutants.
- a 13-fold decrease in N-acetyllactosamine (LacNAc) expression from normal lung tissues to grade one adenocarcinoma specimens, suggesting LacNAc may serve as an early diagnostic marker for lung cancer that currently lacks early diagnostic approaches.
From the above studies, we discovered that H. pylori α1-3-fucosyltransferase possesses a previously unappreciated donor substrate scope: it is able of transferring biopolymers, such as a full-length antibody, to LacNAc in the glycocalyx of live cells when the antibody is conjugated to the enzyme’s natural donor substrate GDP-fucose. This transformation is specific and quantitative, taking place at four degrees within a few minutes with little interference to cells’ endogenous functions. To the best of our knowledge, this is the first example of a promiscuous glycosyltransferase that can accept an unnatural nucleotide sugar donor (molecular weight = 589 kD) conjugated to a protein (molecular weight = 150 kD).
Exploiting the unprecedented substrate scope of this enzyme, we developed a simple and cost-effective technique to fabricate antibody-cell conjugates (ACCs) using primary immune cells and NK-92MI cells that are currently undergoing clinical trials as an “off-the-shelf therapeutic” for cancer immunotherapy. NK-92MI cells do not express Fc receptors that are required for antibody-dependent cell-mediated cytotoxicity (ADCC) to induce specific cell lysis, which significantly limited their therapeutic applications. By incorporating the HER2-specific antibody Herceptin onto the cell-surface of NK-92MI cells via the chemoenzymatic approach, we endowed the modified NK-92MI cells with specific targeting capabilities. The resulting Herceptin-NK-92MI conjugates exhibit remarkably enhanced activities to induce the lysis of HER2+ cancer cells both ex vivo and in vivo in a human tumor xenograft model.
From the above studies, we developed a strong interest in immunology. We realized that we are capable of addressing challenging problems in immunology from the aspect of a chemist. Particularly, we found ourselves in a unique position to contribute new tools that would propel the field of immunobiology, and this has been the theme of our group at Scripps for the past seven years.
The current research in the Wu laboratory integrates synthetic chemistry with glycobiology to explore the cellular and molecular mechanisms that control immune responses toward cancer and human pathogens. We are particularly interested in developing chemoenzymatic tools to study these processes and engineer the cell surface of immune cells for therapeutic applications.
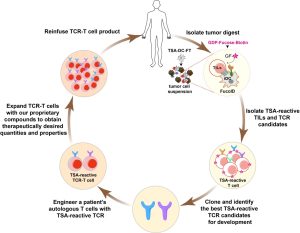
Our long-term goal is to develop new chemical tools and implement them to address the following unsolved problems in cancer immunology: What are the compositions and properties of tumor-specific antigen (TSA)-reactive and bystander tumor-infiltrating lymphocytes (TILs) in the tumor microenvironment? How to address the on-target, off-tumor toxicity of chimeric antigen receptor (CAR)-T cells? Can we produce T cells with therapeutically desired quantities and stem-cell-like properties during ex vivo expansion? How to overcome the immunosuppressive tumor microenvironment to treat solid tumors?
Knowledge gleaned from the exploration of the above four challenging problems would further our understanding of basic tumor immunology. Moreover, tools developed in these studies would facilitate the transformation of T cell-based pre-clinical candidates, including those based on TILs, genetically engineered T cells, and bispecific T-cell engagers, into next-generation therapeutical agents.
References:
Yang, Z.; Grande, G.; Hou, Y.; Wang, C.; Shi, Y.; Lerner, R. A.; Wu, P. Enhancing the Anti-tumor Efficacy of Bispecific T cell Engagers via Cell Surface Glycocalyx Editing. bioRxiv 2022, doi: 10.1101/2022.05.22.492978. https://www.biorxiv.org/content/10.1101/2022.05.22.492978v1
Hong, S.; Yu, C.; Wang, P.; Shi, Y.; Cao, W.; Cheng, B.; Chapla, D. G.; Ma, Y.; Li, J.; Rodrigues, E.; Narimatsu, Y.; Yates, J. R.; Chen, X.; Clausen, H.; Moremen, K. W.; Macauley, M. S.; Paulson, J. C.; Wu, P. Glycoengineering of NK Cells with Glycan Ligands of CD22 and Selectins for B-Cell Lymphoma Therapy. Angewandte Chemie (International Ed. in English) 2021, 60 (7), 3603 – 3610. https://pubmed.ncbi.nlm.nih.gov/33314603/
Hong, S.; Yu, C.; Rodrigues, E.; Shi, Y.; Chen, H.; Wang, P.; Chapla, D. G.; Gao, T.; Zhuang, R.; Moremen, K. W.; Paulson, J. C.; Macauley, M. S.; Wu, P. Modulation of Siglec-7 Signaling via in Situ-Created High-Affinity Cis-Ligands. ACS Central Science 2021, 7 (8), 1338 – 1346. https://pubmed.ncbi.nlm.nih.gov/34471678/
Liu, Z.; Li, J. P.; Chen, M.; Wu, M.; Shi, Y.; Li, W.; Teijaro, J. R.; Wu, P. Detecting Tumor Antigen-Specific T Cells via Interaction-Dependent Fucosyl-Biotinylation. Cell 2020, 183 (4), 1117 – 1133.e19. https://pubmed.ncbi.nlm.nih.gov/33096019/
Hong, S.; Sahai‐Hernandez, P.; Chapla, D. G.; Moremen, K. W.; Traver, D.; Wu, P. Direct Visualization of Live Zebrafish Glycans via Single‐Step Metabolic Labeling with Fluorophore‐Tagged Nucleotide Sugars. Angewandte Chemie (International Ed. in English) 2019, 58 (40), 14327 – 14333. https://pubmed.ncbi.nlm.nih.gov/31295389/
Li, J.; Chen, M.; Liu, Z.; Zhang, L.; Felding, B. H.; Moremen, K. W.; Lauvau, G.; Abadier, M.; Ley, K.; Wu, P. A Single-Step Chemoenzymatic Reaction for the Construction of Antibody-Cell Conjugates. ACS Central Science 2018, 4 (12), 1633 – 1641. https://pubmed.ncbi.nlm.nih.gov/30648147/
Schneider, M.; Kumar, V.; Nordstrøm, L. U.; Feng, L.; Takeuchi, H.; Hao, H.; Luca, V. C.; Garcia, K. C.; Stanley, P.; Wu, P.; Haltiwanger, R. S. Inhibition of Delta-Induced Notch Signaling Using Fucose Analogs. Nature Chemical Biology 2018, 14 (1), 65 – 71. https://pubmed.ncbi.nlm.nih.gov/29176671/
Rouhanifard, S. H.; López-Aguilar, A.; Wu, P. CHoMP: A Chemoenzymatic Histology Method Using Clickable Probes. ChemBioChem 2014, 15 (18), 2667 – 2673. https://pubmed.ncbi.nlm.nih.gov/25403986/
Besanceney-Webler, C.; Jiang, H.; Zheng, T.; Feng, L.; Soriano del Amo, D.; Wang, W.; Klivansky, L. M.; Marlow, F. L.; Liu, Y.; Wu, P. Increasing the Efficacy of Bioorthogonal Click Reactions for Bioconjugation: A Comparative Study. Angewandte Chemie (International Ed. in English) 2011, 50 (35), 8051 – 8056. https://pubmed.ncbi.nlm.nih.gov/21761519/
Zheng, T.; Jiang, H.; Gros, M.; del Amo, D. S.; Sundaram, S.; Lauvau, G.; Marlow, F.; Liu, Y.; Stanley, P.; Wu, P. Tracking N-Acetyllactosamine on Cell-Surface Glycans in Vivo. Angewandte Chemie (International Ed. in English) 2011, 50 (18), 4113 – 4118. https://pubmed.ncbi.nlm.nih.gov/21472942/
Soriano Del Amo, D.; Wang, W.; Jiang, H.; Besanceney, C.; Yan, A. C.; Levy, M.; Liu, Y.; Marlow, F. L.; Wu, P. Biocompatible Copper(I) Catalysts for in Vivo Imaging of Glycans. Journal of the American Chemical Society 2010, 132 (47), 16893 – 16899. https://pubmed.ncbi.nlm.nih.gov/21062072/
Wang, W.; Hu, T.; Frantom, P. A.; Zheng, T.; Gerwe, B.; del Amo, D. S.; Garret, S.; Seidel, R. D.; Wu, P. Chemoenzymatic Synthesis of GDP- L -Fucose and the Lewis X Glycan Derivatives. Proceedings of the National Academy of Sciences 2009, 106 (38), 16096 – 16101. https://pubmed.ncbi.nlm.nih.gov/19805264/